News
October 3, 2024
June 6, 2024
Expert Reviews and Publications
Roulier, M. et al. 2024. Journal of Mass Spectrometry, 59:e5005.
“This article compares five published methods for the separation and preconcentration of 226Ra in drinking waters based on chromatographic and extraction resins prior to its analysis by ICP-MS. We evaluated the turnaround time, generated wastes, and final cost of each protocol as the economic aspect can be an important criterion when selecting a method, particularly for sustainable environmental monitoring.”
Of the five published methods, IBC’s AnaLig® Ra-01 [a first-generation Molecular Recognition Technology™ (MRT™) resin for radium purification] was shown to be advantageous in key waste and cost metrics:
- Lowest Waste Generation. Waste per processed sample was between 3.5 to 18.7 times less than the other methods
- Lowest Cost. Cost per processed sample was 1.75 to 3.3 times lower than the other methods
Izatt, R.M. 2022
A summary of five case studies in which Molecular Recognition Technology™ was used to separate radioisotopes:
- Pd-103 Separation and Recovery in Brachytherapy
- Selective separation and recovery of Cs-137 from nuclear wastes at the U.S. Department of Energy Savannah River Site in South Carolina
- Demonstration of selective separation and recovery of Cs-137 and Tc-99 from nuclear wastes stored at Hanford, Washington dating to the World War II period
- Selective separation of radioactive Cs-134 and Cs-137 from fly ash derived from incinerated material related to the tsunami occurring after the 2011 earthquake in Japan
- Demonstration of the selective separation and recovery of radioactive Cs-137 and Sr-90 from contaminated seawater in Fukushima Harbor, at the site of the 2011 tsunami
Izatt, R.M. 2022
“Molecular Recognition Technology™ (MRT™) processes have proven capabilities in the separation and recovery of a variety of elements found in decay products of U, such as Pu, Th, Ra, and Bi [27,30-33], as well as elements produced in fission of U, such as Cs, Sr, Tc, Pu, Am, Pd, and I [27,28,30,34].
Coha, B. et al. 2021. Talanta, 225, 121959.
“These highly selective materials for Sr have enabled the development and implementation of automated and miniaturised procedures, which allow more rapid procedures than conventional ones, decrease labour costs, reduce waste, and most importantly, decreases analyst exposure to radiological dose.”
“If the time necessary to obtain pure Sr fractions is compared, roughly two and a half days are required for the method using a combination of anion exchange resins and nitric acid in an alcohol solution around the proposed method reduces this time even further to 1–5 h, as well as additionally separating and determining Pb and Ba. Chemical recoveries were also checked by spiking the samples with Sr-85; the results showed that recovery was (99 ± 3) % in all cases.”
“Such solid phase extraction systems, based on Molecular Recognition Technology™ (MRT™) are highly discriminating; they have the ability to distinguish and separate target ions even if they are present in trace amounts in solutions containing higher concentrations of ions with similar chemical properties.”
Thakur, P. et al. 2021. Journal of Environmental Radioactivity, 228, 106522.
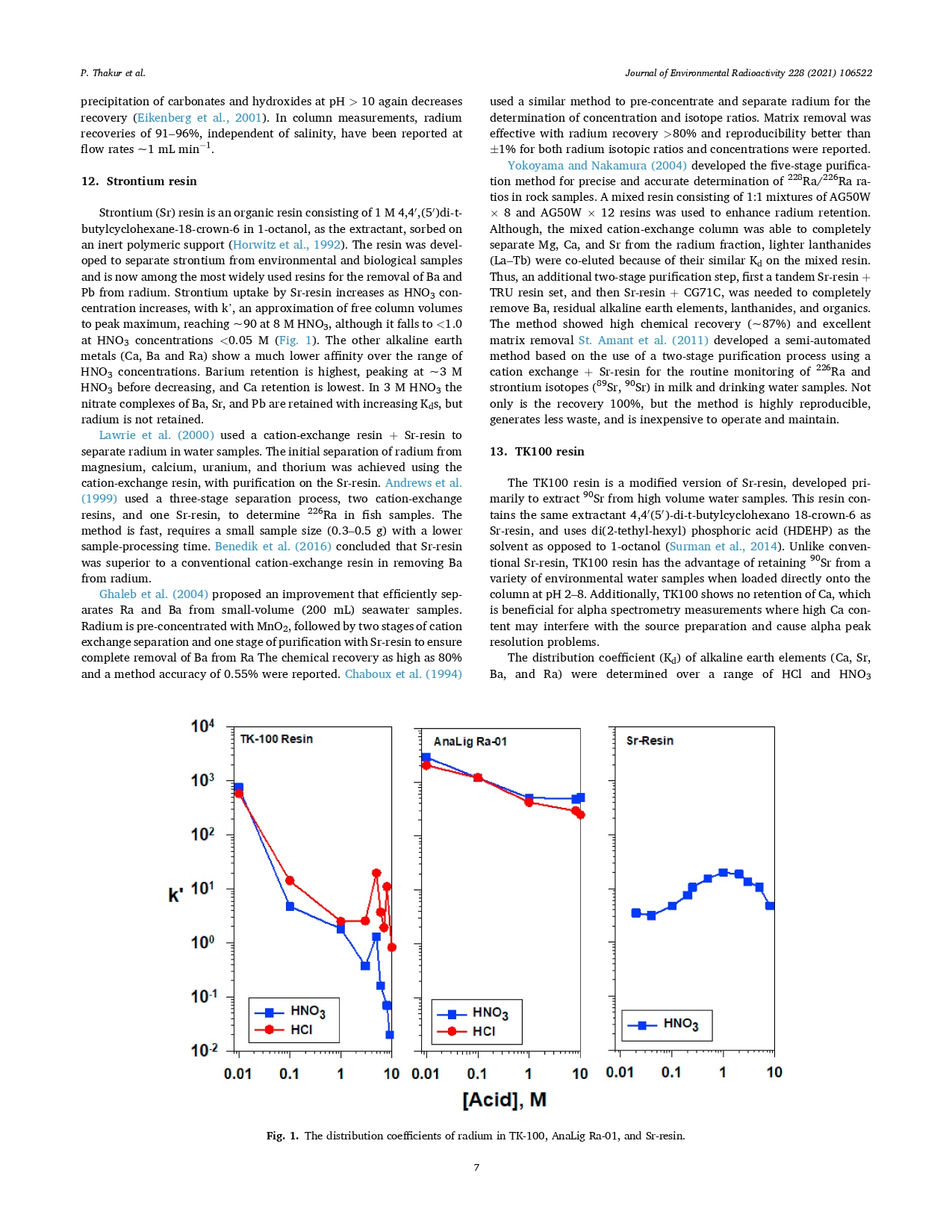
Figure 1 shows AnaLig® Ra-01 [a first-generation Molecular Recognition Technology™ (MRT™) resin for radium purification] to have far superior distribution coefficients compared to other commercially available resins. “Radium was strongly retained by the AnaLig®Ra-01 resin over the entire range of acid concentrations (0.01–10 M HCl and HNO3). Distribution coefficients (Kd) were >250 L/kg over the entire acid range in both HCl and HNO3 media.”
Sections 14 and 15 describe the use of IBC resins, such as AnaLig® Ra-01, however, a few points of clarification are needed. Section 14 claims Ra separation won’t work in HCl which is not correct. HCl and its binding with Pb and Tl do prevent those from binding, but Ra will bind.
Also, Section 15 claims certain water samples work and some don’t for Ra on Empore. The lack of anions is the reason some water samples need acid addition before Ra binding, but other reasons are incorrectly given such as other cations being at large excess preventing binding which is incorrect.
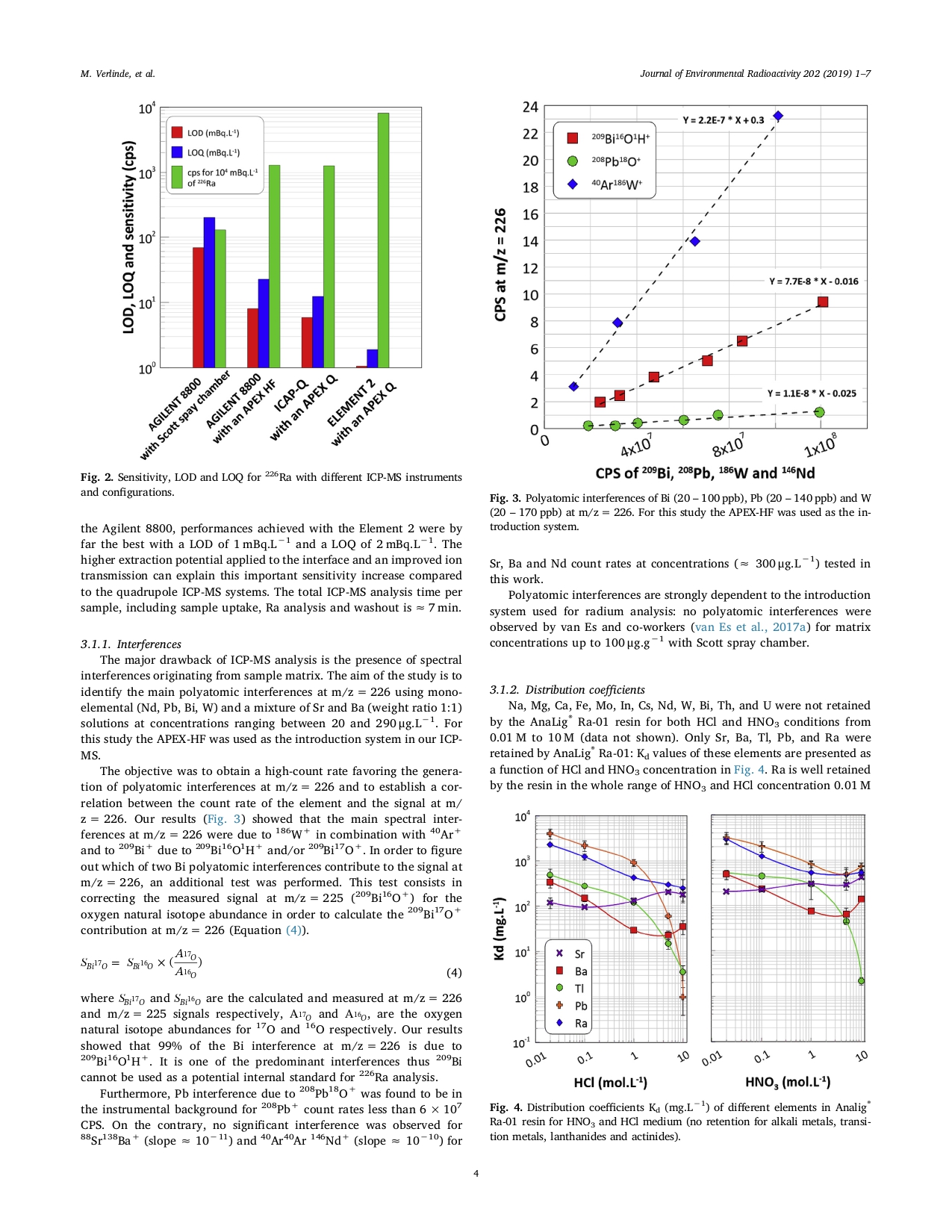
Verlinde, M. et al. 2019. Journal of Environmental Radioactivity, 202, 1-7.
This paper demonstrates the excellent recovery and selectivity of, AnaLig® Ra-01, a first-generation Molecular Recognition Technology™ (MRT™) resin for radium purification.
“This work presents a new fast 226Ra separation protocol using the Analig® Ra-01 resin [a first-generation Molecular Recognition Technology™ (MRT™) resin for radium purification]. Batch and column results showed high specificity for 226Ra in a wide range of acid concentrations to 0.01 M from 10 M. Radium competitor elements were found to be only Sr, Ba, Tl and Pb. The determination of distribution coefficients allowed identifying conditions for their elution before radium fraction. Radium elution was performed with 0.12 M NTA. Ba coelutes with Ra, however Ba itself cannot interfere with 226Ra in ICP-MS analysis. The protocol was tested using a synthetic multi-element solution doped with 226Ra and our result showed that the sample matrix and the interfering elements were efficiently separated from Ra. Furthermore, radium recovery for the synthetic multi-element solution was found to approach 100%.”
DiPrete, D. et al. 2018. Talanta, 225, 318:1663–1670.
“Waste cleanup efforts currently underway at the Savannah River Site have created a need to characterize Radium-226 levels in the various high activity waste matrices currently in Site inventories. The traditional method our laboratory used for analyzing Ra-226 in higher activity matrices was based on classic cation exchange methodology. Radiochemical separations were often initiated in remotely operated shielded analytical cells followed by additional hands-on separations in radiological hoods. Methodology based on IBC [A]dvanced [T]echnologies SuperLig 640 extractant, mounted in 3M Empore filter media has been developed to stream[l]ine the radium analyses.”
Ito, S. et al. 2018 Prog. Theor. Exp. Phys., 091H01
“High-purity germanium (HPGe) detectors are widely used for the measurement of low concentrations of radioactivity. For the Super-Kamiokande Gadolinium project, the concentration of radium in Gd2(SO4)3·8H2O is determined by using HPGe detectors. The amount of the sample is generally limited by the size of the sample space of an HPGe detector. This leads to limitation of the detector efficiency. A new method of chemical extraction using molecular recognition resin was developed to minimize this problem. Using the developed method, radium could be extracted from Gd2(SO4)3·8H2O and concentrated to a smaller amount of the resin at a recovery rate of 81%±4%, which was estimated by using barium as a recovery monitor. This made it possible to set the sample closer to an HPGe crystal and reduce the self-screening effect, resulting in increased detection efficiency for an HPGe detector. The method developed is reported in this letter.”
Izatt, R.M. et al. 2017 In Beach, E.S., Kundu, S., (Eds.), Handbook of Green Chemistry Volume 10 – Tools for Green Chemistry. (Anastas, P.T., (Ed.). Handbook of Green Chemistry Series.) Wiley-VCH, Weinheim, Germany, pp. 189-240
Chapter 9 in the Handbook of Green Chemistry describes many applications of Molecular Recognition Technology™ (MRT™). Sections related specifically to radionuclide separations include:
9.3.2.7 Purification of 103Palladium for Use in Brachytherapy
9.3.14 Radionuclide Remediation
9.4.2 Radionuclides
Bruening, R.L. et al. 2016 Waste Management (WM) 2016 Conference, Phoenix, AZ, March 6-10
“This effort was part of a demonstration project to investigate methods for removal of Sr-90 and Cs-134/Cs-137 from 160,000 cubic meters of seawater impounded at Fukushima, Dai’ichi, Japan harbor. SuperLig® resins [first-generation Molecular Recognition Technology™ (MRT™) resins] can be regenerated by elution with small volumes of simple acid solutions. SuperLig® resins have a high tolerance to radiation, with their performance not being degraded by doses of 1.0E+9 Rad.”
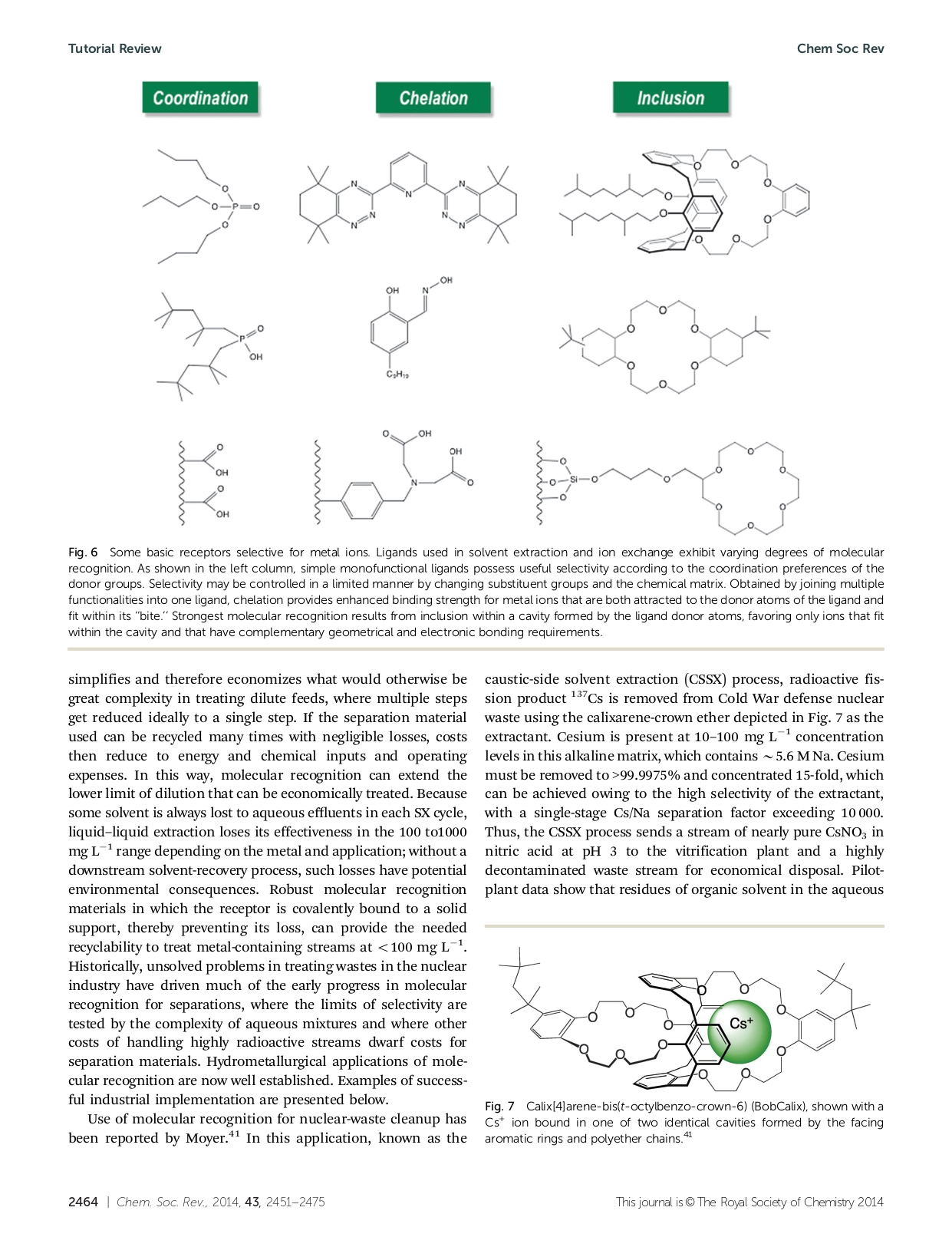
Izatt, R.M. et al. 2014 Chemical Society Reviews, 43, 2451-2475
“Use of molecular recognition for nuclear-waste cleanup has been reported by Moyer.41 In this application, known as the caustic-side solvent extraction (CSSX) process, radioactive fission product Cs-137 is removed from Cold War defense nuclear waste using the calixarene-crown ether depicted in Fig. 7 as the extractant. Cesium is present at 10–100 mg L-1 concentration levels in this alkaline matrix, which contains ~5.6 M Na. Cesium must be removed to >99.9975% and concentrated 15-fold, which can be achieved owing to the high selectivity of the extractant, with a single-stage Cs/Na separation factor exceeding 10,000. Thus, the CSSX process sends a stream of nearly pure CsNO3 in nitric acid at pH 3 to the vitrification plant and a highly decontaminated waste stream for economical disposal.”
Izatt, S.R. et al. 2009 Waste Management Conference, Phoenix, AZ, March 1-5
“This paper reviews the application of Molecular Recognition Technology™ (MRT™), a highly selective separations technology, as an alternative to existing uranium extraction and recovery technologies used in the uranium mining and refining industries. Extraction of deleterious elements from acid mine drainage and aqueous neutral and near neutral mine streams is also discussed as well as applications for MRT in the separation, extraction, recovery and analysis of target elements and ions in power plant and other waste streams and the purification of isotopes such as Pd-103 for brachytherapy.”
Izatt, S.R. et al. 2003 WM (Waste Management) 2003 Conference, Tucson, AZ, February 23-27
“This paper describes case studies of the use of commercial products developed by IBC and currently in industrial use for the separation of radionuclides from different waste types. Uses of the following [first-generation Molecular Recognition Technology™ (MRT™) resins] will be described: SuperLig® 644 for Cs removal and SuperLig® 639 for Tc removal; SuperLig® 620 and SuperLig® 640 prepared at IBC and used in Rad Disks manufactured by 3M for the selective separation and analysis of Sr and Ra, respectively; and MacroLig® 209 (BOB Calix C6) used in the cesium solvent extraction (CSSX) Process at Savannah River, South Carolina. Separations of several other anions and cations of interest to the nuclear industry will be noted.”